One of our specialties in the Origins and Habitability Laboratory is making artificial hydrothermal vents in the lab. These experiments are done to simulate specific types of vent chemistries on modern Earth, early Earth, early Mars, and ocean worlds. By controlling the conditions in lab - such as ocean chemistry, hydrothermal fluid composition, and atmosphere - we can simulate a variety of types of vent that are relevant to astrobiology and origin of life.
This page contains some examples of the artificial vent experiments we do; see my Publications page to learn more!
Iron Hydroxide Chimneys represent hydrothermal minerals that would have precipitated on early Earth; these minerals may be important for prebiotic chemistry since they can react with organics.
We grow these chimneys under a nitrogen atmosphere since iron is very redox sensitive, and we are simulating the early Earth's atmosphere before atmospheric oxygen. The hydrothermal fluid is alkaline and contains hydroxide (OH-) ions; the ocean simulant here contains a combination of ferrous and ferric chloride (Fe2+ and Fe3+). The slow injection of hydrothermal fluid into the ocean simulant with a syringe pump causes the formation of the chimney over several hours.
Read more here and here about iron hydroxide minerals reacting with organics!
(Image taken by former OHL intern Eduardo Martinez)
Magnesium Silicate Chimneys simulate a basalt-hosted alkaline hydrothermal system such as the Strytan Hydrothermal Field in Iceland. Here, alkaline silica-rich hydrothermal fluids feed into seawater, and the hydrothermal silica precipitates with seawater Mg to form chimneys containing Mg-silicate minerals (saponite). The ocean simulant here contains seawater salts including Mg, and the hydrothermal simulant is alkaline and contains silicate. Interestingly we found that with this chemistry, we had to add a layer of mesh on the top of the inlet where the hydrothermal fluid comes in, to give the minerals somewhere to nucleate and then the chimney will form.
The Strytan site is an example of a "shallow vent" where hydrothermal chemistry can interact with shallow waters and atmosphere processes. We make these lab chimneys so that we can study their ability to support life on other worlds, as well as their properties as interesting environments for prebiotic chemistry.
Read more about shallow vents and silicate chimney experiments!
(Image taken by former OHL intern Julia Chavez)
Iron-Magnesium-Silicate Chimneys are similar to the Mg-silicate example above, except here we add iron (Fe) as well to the ocean simulant. This represents early Earth's iron-rich ocean that existed before atmospheric oxygen, and the chimneys simulate early Earth hydrothermal vents in silica-rich systems. See here for the published study.
These chimneys simultaneously precipitate Fe-silicates (green) and Mg-silicates (white); the "patchiness" of the chimney is important since it means different parts of the chimney would contain different minerals, and maybe be able to drive different types of chemical reactions. Some of our chimneys including this one can also be removed from the ocean simulant for further analysis.
(Images taken by former OHL interns Julia Chavez and Nancy Carman)
Iron Sulfide Chimneys form by injecting alkaline, sulfide-rich hydrothermal solution into an ocean simulant containing dissolved iron. This kind of chimney represents metal-sulfide containing vents on early Earth, and is particularly interesting for origin of life studies since the minerals formed in this kind of chimney can react with organics to drive various reactions.
These sulfide lab chimneys are very fragile and oxygen-sensitive and thus can be challenging to handle. They fall apart easily if the experiment is perturbed, and if we try to remove the ocean the chimney often collapses so it can't be collected as easily for analysis. This is why we have developed various other methods of forming chimney precipitates in gradients for study of the mineralogy and reactivity of the solids: including "fuel cell" experiments that form the chimney as a flat membrane (publication), and "scaffolds" where the chimney is grown inside a structural support (publication).
(Image taken by Laurie Barge)
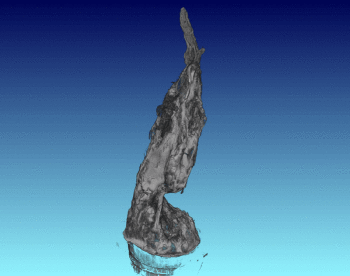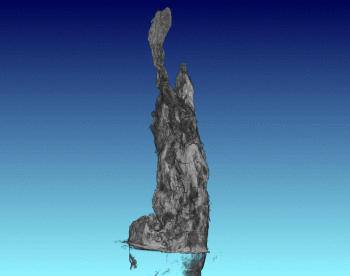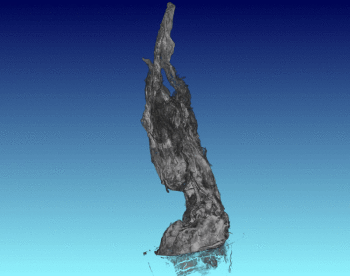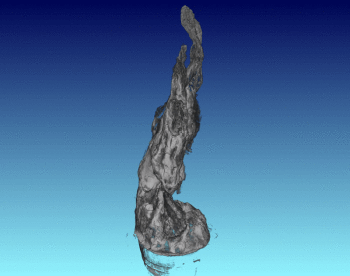
3D Structure of Artificial Chimneys
We have studied the 3D and interior structure of our lab-grown hydrothermal chimneys using X-ray CT scanning. This was done by growing an iron hydroxide chimney as above, then removing the ocean simulant and encasing the chimney in resin.
We found that the chimney structure has a porous interior, and the inside structure is made of intertwined fluid flow conduits and pores with a high internal surface area. This means that organic or prebiotic reactions going on inside these vents could concentrate molecules inside the pores, and also move molecules from part of the chimney to another so reactions could continue under different conditions.
See the published study for more!
Electrochemistry of Artificial Vents
Vents are electrochemical systems since they contain gradients between hydrothermal fluids and seawater that can generate electrical potential (voltage). We can measure the voltage generated in our artificial vents by growing the chimney around a wire, and placing another wire in the ocean simulant. Then as the chimney grows it generates an electrical potential that we can measure.
In one study, we electrically linked together several chimney experiments and found that the chimneys generated enough potential and current to light an LED. This is a lab demonstration of the electrical energy generated by chimneys, but shows how hydrothermal vents on early Earth and other worlds might also generate similar energy, and in a geological setting that energy could be used to support life or drive organic reactions.
(Photo taken by Bethany Theiling)